3D Printed HDR Surface Brachytherapy Applicator for Treatment of Kaposi’s Sarcoma
Hôpital Charles-Le Moyne Patient Case Study
This case is an example of how Adaptiiv’s 3D Brachy software can be used to design a patient-specific, 3D printed high dose rate (HDR) surface brachytherapy applicator to treat Kaposi’s sarcoma of the hand. Personalized 3D printed surface brachytherapy applicators provide consistent contact between the skin and applicator, resulting in increased precision of dose distribution, particularly for traditionally challenging sites with extreme curvature.
This case is also available in French. Read the French version here.
Patient History
A 79-year-old male patient presented with Mediterranean Kaposi’s sarcoma, which resulted in a lesion on his right hand.
Description
HDR surface brachytherapy has many advantages. It can deposit a significantly higher dose within a tumor while sparing adjacent normal structures compared to external beam radiation therapy techniques. These techniques can require irradiating a larger volume of healthy tissue to sufficiently cover the malignancy, thus increasing the potential for secondary effects. Surface brachytherapy is best for lesions located at or just below the skin surface since the dose fall-off is significant at depths greater than 5 mm from the skin surface.
Design & Fabrication
The setup for the initial scan is shown in Figure 1. The physician placed a CT marker wire to delineate the lesion and a custom Vac-Lok was made for the patient to provide better comfort and immobilization.
Images were imported to RayStation (RaySearch Laboratories, Stockholm, Sweden) to create an external contour of the patient and a 1.5 cm thick applicator around the region of interest. To improve positioning reproducibility, the applicator was designed to be larger than the lesion and utilized the fingers as an anatomical landmark. The external body and applicator structures are shown in Figure 2.
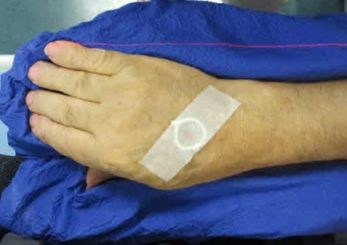
Figure 1: Patient setup for a CT scan performed using a Siemens Somatom Sensation Open. Images were acquired at 2.5 mm slice thickness.
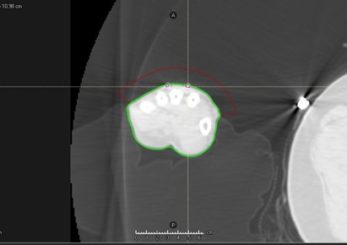
Figure 2: Structures of interest created in the TPS. The external body (green), the wires (pink), and the applicator (red) can be seen.
DICOM data structures and images were imported to Adaptiiv’s 3D Brachy software. The software’s automatic tunnel generation tool was used to create 9 trajectories, 5 mm from the surface of the patient’s external body contour. The trajectory algorithm enforcing inter-slice distances opposed to inter-trajectory distances was chosen in the axial direction using a 10 mm inter-trajectory distance and a 1.6 mm tunnel radius. Step 1 of the trajectory setup process is shown in Figure 3.
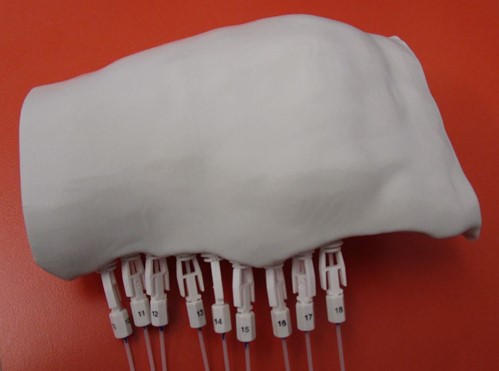
The applicator design was exported as an STL file from Adaptiiv’s 3D Brachy software and printed with a Creality Ender 3 3D printer (Creality 3D Technology Co., Shenzen, China). Flexible 4F 240 mm catheters were inserted through the tunnels and set in place with buttons to allow the HDR source to travel reliably through the applicator. The 3D printed applicator with the catheters installed is shown in Figure 4.
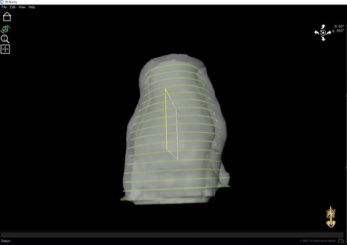
Figure 3: The initial trajectory setup for the applicator. The inter-trajectory distance was 10 mm, with the inter-slice distance enforced.
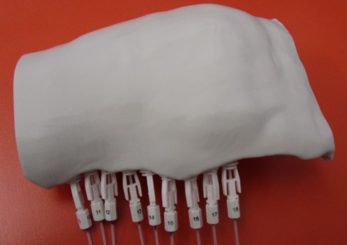
Figure 4: The final 3D printed applicator installed with the catheters and buttons.
A second CT scan of the patient with the applicator and a CT marker wire was performed to ensure proper fit, verify print quality, create reference marks for daily setup, and enable catheter reconstruction for treatment planning.
Thick slabs of Superflab (Radiation Products Design, Albertville, Minnesota) bolus were placed on the applicator to ensure stability before the second CT. The physician drew the clinical treatment volume (CTV) and planned using the Oncentra Brachy TPS (Elekta, Stockholm, Sweden). The planning results can be seen in Figure 5.
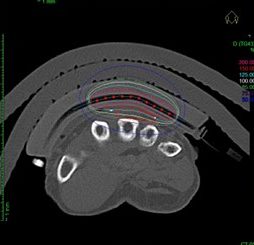
Figure 5: Treatment planning results for the customized applicator. The CT showed that the applicator conformed well to the patient’s surface with minimal air gaps.

Figure 6: Applicator on the patient with Superflab placed on top for additional stability.
Dose Calculation & Verification
Using the AAPM TG-43 dose algorithm, a treatment plan was developed to ensure 100% coverage of the CTV by the 85% isodose without having more than a 6% volume for the 125% isodose at the skin surface. The planned dose was 30 Gy in 10 fractions BID for 5 consecutive days with a minimum 6 hour gap between fractions. Treatment time was 110.5 seconds per fraction. Figure 6 shows the final treatment position.
Summary
- 3D printed HDR surface brachytherapy applicators designed in Adaptiiv’s 3D Brachy software can be used to treat Kaposi’s sarcoma of the hand.
- The patient-specific applicator provided an excellent fit to the patient’s contour, supporting consistent contact between the applicator and lesion with reproducible setup.
- Hollow catheter trajectories were easily designed with user-defined separation and stand-off distances for challenging geometries using Adaptiiv’s 3D Brachy software tunnel generation tool.
- The prescribed dose was optimized to the CTV while minimizing the skin surface dose.