Currently 3D Printing in Radiation Oncology
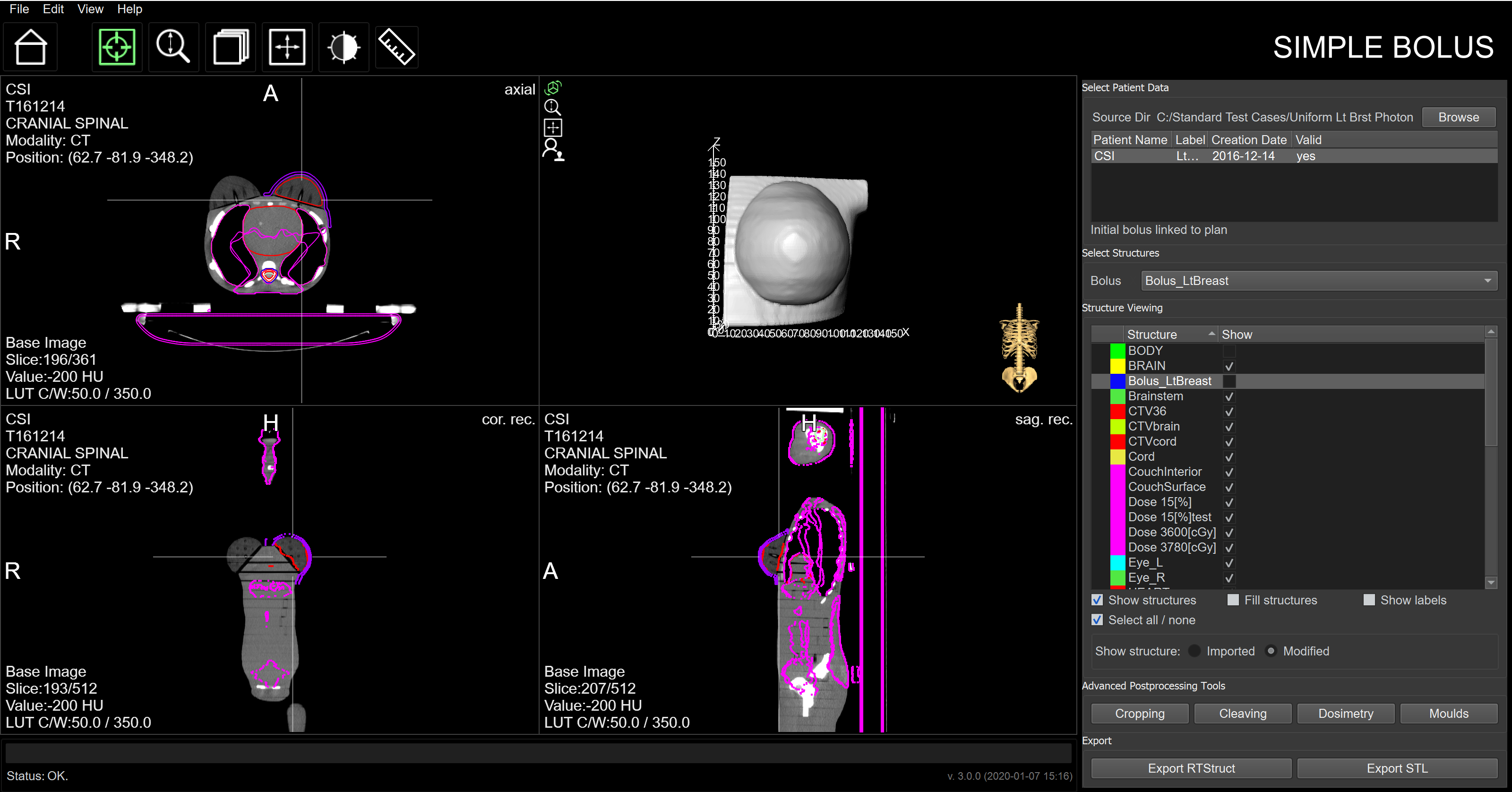
The Advantages of Using Adaptiiv Software
Since you are already familiar with the advantages of using 3D printing in RT to fabricate high quality custom accessories, learn how using our regulatory cleared software in conjunction with your 3D printing network can further enhance clinical efficiencies.
Regulatory Clearance
Using regulatory cleared software provides confidence.
Our 3D printing software solution has FDA 510(k) clearance, a CE Mark, TGA approval, and an ISO 13485 compliant QMS.
ROI of Existing 3D Printing Network
Optimize the use of existing assets.
Increase your center’s throughput of custom bolus and/or brachytherapy applicators on demand via an automated design and fabrication process.
Design and print patient-specific RT accessories at a fraction of the cost of conventional accessories such as the Freiburg Flap and bill for reimbursement (where applicable).
Quality Control And Assurance
Reduce risk.
Our software provides clinics with a fully validated, turnkey solution to improve accuracy, reproducibility, and provide repeatable, standardized treatment for even the most complex cases.
By integrating directly with RT workflows, users can customize an accessory directly within our software and export it back to the TPS for dose and plan verification. This minimizes the risk of delivering inadequate RT treatment and prevents potential treatment delays compared to more complicated DIY solutions.
Personalized Treatment Made Easier
Implementation of 3D printing into the RT workflow is simple and efficient.
With our software, accessory design can be completed without the need for the patient to be present if existing CT data is on file, saving patients an extra visit during treatment. Time is also saved during patient set up by eliminating the need for labor-intensive manual adjustments using wax, excessive taping, or using wet gauze to create an acceptable fit.
Users can also improve the overall efficiency of creating RT accessories – what could take more than 30 hours to manually design and validate a complex bolus or brachytherapy applicator can be achieved in a matter of minutes when using our software.
Validation
Printers and filament materials have been validated and verified for use in RT.
We have invested more than 10,000 hours properly validating 3D printers and filament materials, ensuring users are in compliance from design to print.
Our validation protocol eliminates guesswork so users can have confidence that the fabricated accessory will result in treatment being delivered as planned.